A Gyógyszerészi Kémiai Tanszék fő kutatási területei:
A Gyógyszerészi Kémia Tanszék sokrétű szerves kémiai és gyógyszerkémiai alapkutatást végez. Antibiotikumkémiai és szénhidrátkémiai kutatási irányai a két előd-kutatócsoport, az MTA Antibiotikumkémiai Kutatócsoport és az MTA Szénhidrátkémiai Kutatócsoport hagyományaira épülnek. Ezek mellett módosított nukleozidok és nukleinsavak szintézisével, valamint és kardiovaszkuláris hatóanyagok kutatásával is foglalkozik.
Aktív kutatások példákkal
Antibiotikum-kémiai kutatások
Lipofil teikoplanin származékok, mint új influenza-ellenes szerek
Zsolt Szűcs, Viktor Kelemen, Son Le Thai, Magdolna Csávás, Erzsébet Rőth, Gyula Batta, Annelies Stevaert, Evelien Vanderlinden, Lieve Naesens, Pál Herczegh, Anikó Borbás: Structure-activity relationship studies of lipophilic teicoplanin pseudoaglycon derivatives as new anti-influenza virus agents
Eur. J. Med. Chem. 2018, 157, 1017-1030.
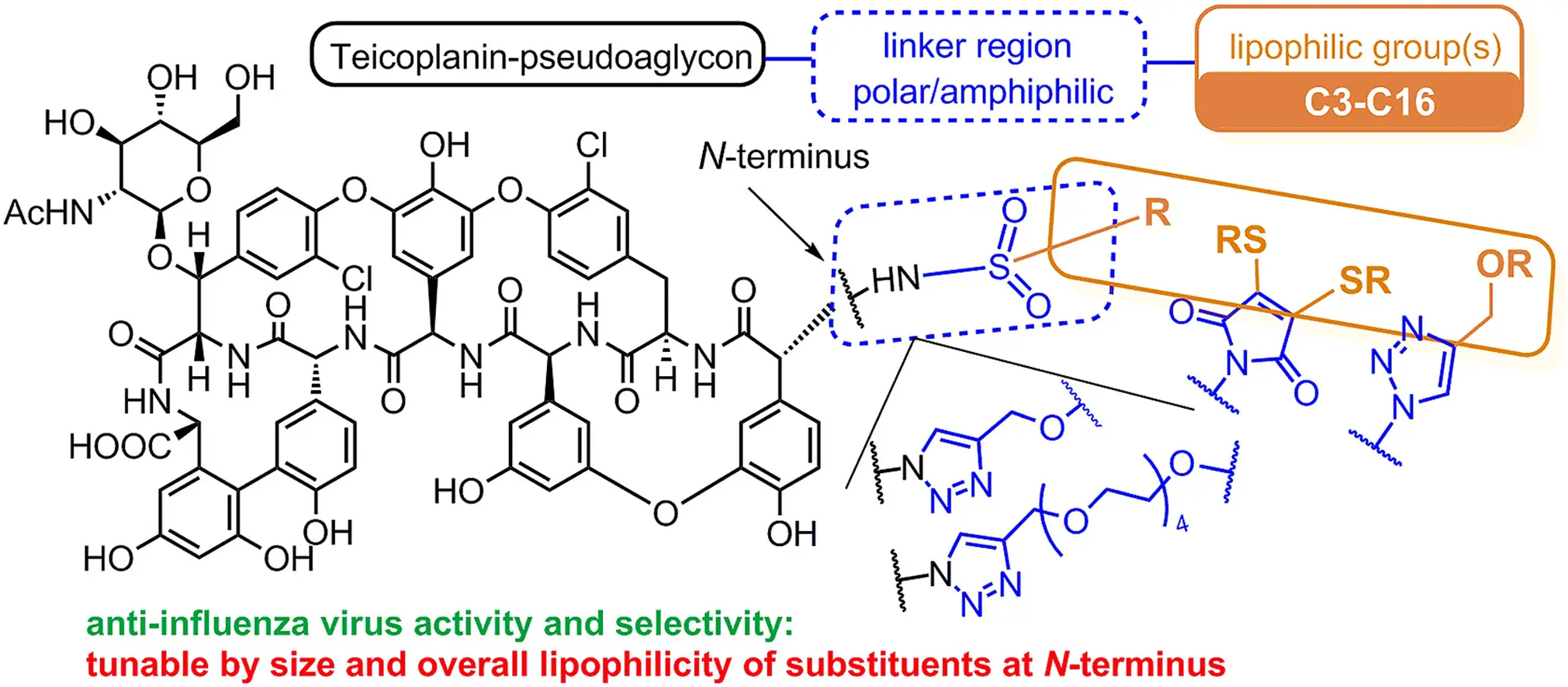
Lipofil oldalláncokat hordozó glikopeptid-antibiotikum származékok szintézise
Csávás, M., Miskovics A., Szűcs, Zs., Rőth E., Nagy, Zs. L., Bereczki, I., Herczeg, M., Batta, Gy., Nemes-Nikodém, É., Ostorházi, E., Rozgonyi, F., Borbás, A., Herczegh, P.: Synthesis and antibacterial evaluation of some teicoplanin pseudoaglycon derivatives containing alkyl- and arylthiosubstituted maleimides
Journal of Antibiotics, 68 (9) 579-585 (2015). DOI: 10.1038/ja.2015.33
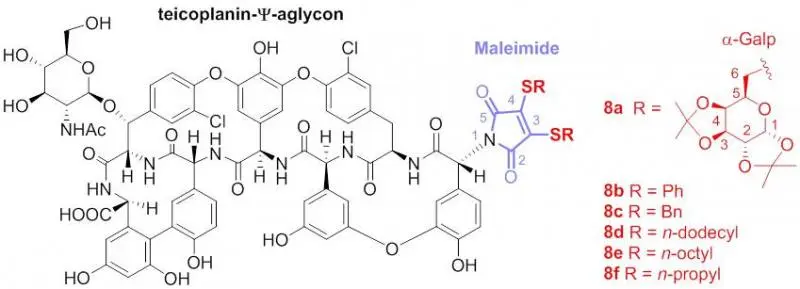
Abstract: Bis-alkylthio maleimido derivatives have been prepared from teicoplanin pseudoaglycon by reaction of its primary amino group with N-ethoxycarbonyl bis-alkylthiomaleimides. Some of the new derivatives displayed excellent antibacterial activity against resistant bacteria.
Antivirális glikopeptid antibiotikumok előállítása
Ilona Bereczki, Máté Kicsák, Laura Dobray, Anikó Borbás, Gyula Batta, Sándor Kéki, Éva Nemes Nikodém, Eszter Ostorházi, Ferenc Rozgonyi, Evelien Vanderlinden, Lieve Naesens, Pál Herczegh: Semisynthetic teicoplanin derivatives as new influenza virus binding inhibitors: Synthesis and antiviral studies
Bioorganic & Medicinal Chemistry Letters 24 (2014) 3251–3254.
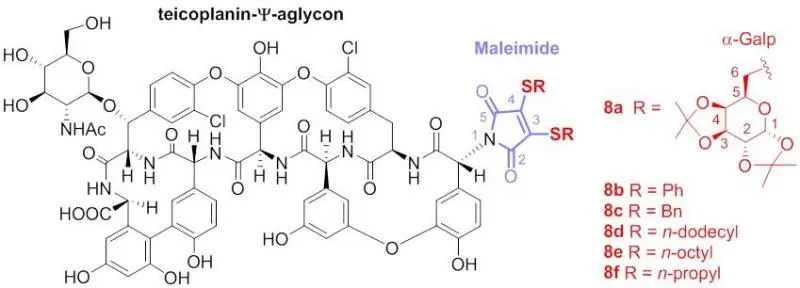
Abstract: In order to obtain new, cluster-forming antibiotic compounds, teicoplanin pseudoaglycone derivatives containing two lipophilic n-octyl chains have been synthesized. The compounds proved to be poor antibacterials, but, surprisingly, they exhibited potent anti-influenza virus activity against influenza A strains. This antiviral action was related to inhibition of the binding interaction between the virus and the host cell. Related analogs bearing methyl substituents in lieu of the octyl chains, displayed no anti-influenza virus activity. Hence, an interaction between the active, dually n-octylated compounds and the lipid bilayer of the host cell can be postulated, to explain the observed inhibition of influenza virus attachment.
Fluorkinolon-karbonsavat tartalmazó kiméra vegyületek előállítása
Szénhidrátkémiai kutatások
Sztereoszelektív 1,2-cis-a-tioglikokonjugáció
Promotion of a Reaction by Cooling: Stereoselective 1,2-cis-a-Thioglycoconjugation by Thiol-Ene Coupling at -80°C
Chem. Eur. J. 2018, 24,4532–4536.

Fotokatalitikus tiol-én addíció vizsgálata és alkalmazása glikokonjugátumok és biológiailag aktív vegyületek szintézisében
Lázár, L., Csávás, M, Herczeg, M, Herczegh, P., Borbás, A.: Synthesis of S-Linked Glycoconjugates and S-Disaccharides by Thiol-Ene Coupling Reaction of Enoses.
Organic Letters, 14, 4650-4653 (2012)
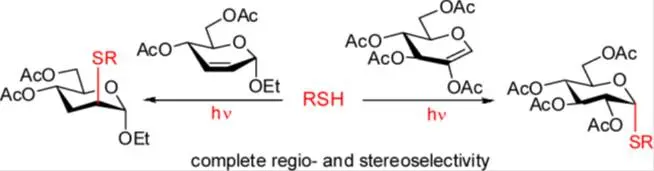
Abstract: Free-radical hydrothiolation of the endocyclic double bond of enoses is reported. Reaction between 2-acetoxy-D-glucal and a range of thiols including amino acid, peptide, glycosyl thiols, and sugars with primary or secondary thiol functions gave S-linked a-glucoconjugates and S-disaccharides with full regio- and stereoselectivity. Addition of glycosyl thiols to a 2,3-unsaturated glycoside also proceeded with good selectivity and afforded a series of 3-deoxy-S-disaccharides.
Multivalens szénhidrátok, mint bakteriális lektinek potenciális ligandumainak előállítása
Gita Jančaříková, Mihály Herczeg, Eva Fujdiarová, Josef Houser, Katalin E. Kövér, Anikó Borbás, Michaela Wimmerová, and Magdolna Csávás: Synthesis of α-L-fucopyranoside-presenting glycoclusters and investigation of their interaction with recombinant Photorhabdus asymbiotica lectin (PHL)
Chem. Eur. J., 2018, 24, 4055 – 4068

Magdolna Csávás, Lenka Malinovská, Florent Perret, Milán Gyurkó, Zita Tünde Illyés, Michaela Wimmerová, Anikó Borbás: Tri- and tetravalent mannoclusters cross-link and aggregate BC2L-A lectin from Burkholderia cenocepacia
Carbohydr. Res., 437, (2017) 1-8.
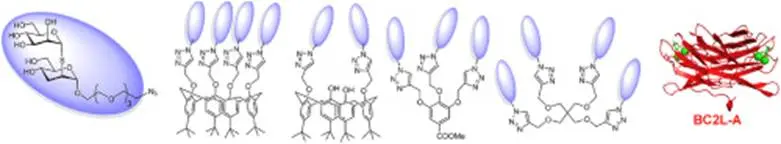
Abstract:The opportunistic Gram-negative bacterium Burkholderia cenocepacia causes lethal infections in cystic fibrosis patients. Multivalent mannoside derivatives were prepared as potential inhibitors of lectin BC2LA, one of the virulence factors deployed by B. cenocepacia in the infection process. An (a1/2)-thiolinked mannobioside mimic bearing an azide functionalized aglycon was conjugated to different multivalent scaffolds such as propargylated calix[4]arenes, methyl gallate and pentaerythritol by azidealkyne 1,3-dipolar cycloaddition. The interaction between the glycoclusters and the mannose binding BC2L-A lectin from B. cenocepacia was examined by isothermal microcalorimetry, surface plasmon resonance, inhibition of yeast agglutination and analytical ultracentrifugation.
Antibiotikum-szénhidrát kiméra vegyületek szintézise
L-iduronsav szintézise és heparinoid pentaszacharidok előállítása
Fruzsina Demeter, Tamás Gyöngyösi, Zsuzsa Bereczky, Katalin E. Kövér, Mihály Herczeg, Anikó Borbás: Replacement of the L-iduronic acid unit of the anticoagulant pentasaccharide idraparinux by a 6-deoxy-L-talopyranose–Synthesis and conformational analysis
Scientific reports 2018, 8 (1), 13736
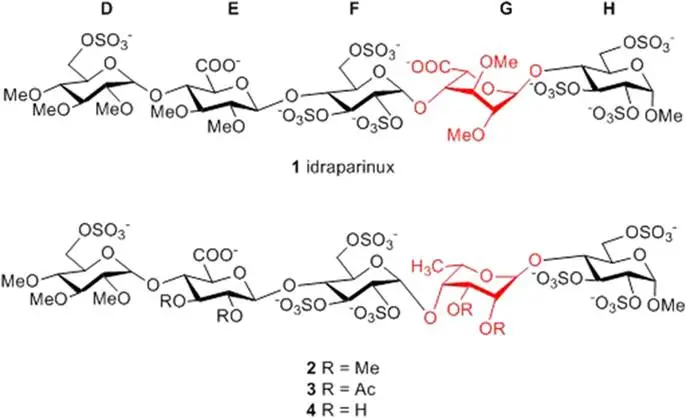
Fruzsina Demeter, Fanni Veres, Mihály Herczeg, Anikó Borbás: Short synthesis of idraparinux by applying a 2‐O‐methyl‐4, 6‐O‐arylmethylene thioidoside as a 1, 2‐trans alpha‐selective glycosyl donor
European Journal of Organic Chemistry, doi: 10.1002/ejoc.201801349
The fully O‐sulfated, O‐methylated, heparin‐related anticoagulant pentasaccharide idraparinux was prepared by a new synthetic pathway in 38 steps using D‐glucose and methyl α‐D‐glucopyranoside as starting materials, with 23 steps for the longest linear route. The L‐idose‐containing GH fragment was obtained by a short and straightforward synthesis whereby a 4,6‐cyclic‐acetal‐protected L‐idosyl thioglycoside bearing a C2‐nonparticipating group was used as the α‐selective glycosyl donor. The novel L‐idose donor was prepared with high chemo‐ and stereoselectivity by hydroboration–oxidation‐based C5 epimerization starting from an orthogonally protected α‐thioglucoside. The assembly of the pentasaccharide backbone was achieved by an F+GH and DE+FGH coupling sequence with full stereoselectivity in each glycosylation step.
Mihály Herczeg, Fruzsina Demeter, Tímea Balogh, Viktor Kelemen, Anikó Borbás: Rapid Synthesis of l‐Idosyl Glycosyl Donors from α‐Thioglucosides for the Preparation of Heparin Disaccharides
Eur. J. Med. Chem. 2018 (25), 3312-3316

Herczeg, M., Lázár, L., Bereczky, Z., Köver, K. E., Timári, I., Kappelmayer, J., Lipták, A., Antus, S., Borbás, A.: Synthesis and Anticoagulant Activity of Bioisosteric Sulfonic-Acid Analogues of the Antithrombin-Binding Pentasaccharide Domain of Heparin.
Chem. Eur. J., 18, 10643-10652 (2012)
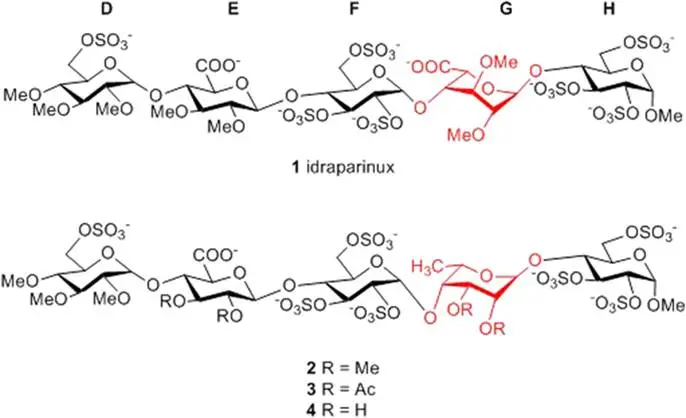
Abstract: Two pentasaccharide sulfonic acids that were related to the antithrombin-binding domain of heparin were prepared, in which two or three primary sulfate esters were replaced by sodium-sulfonatomethyl moieties. The sulfonic-acid groups were formed on a monosaccharide level and the obtained carbohydrate sulfonic-acid esters were found to be excellent donors and acceptors in the glycosylation reactions. Throughout the synthesis, the hydroxy groups to be methylated were masked in the form of acetates and the hydroxy groups to be sulfated were masked with benzyl groups. The disulfonic-acid analogue was prepared in a [2+3] block synthesis by using a trisaccharide disulfonic acid as an acceptor and a glucuronide disaccharide as ma donor. For the synthesis of the pentasaccharide trisulfonic acid, a more-efficient approach, which involved elongation of the trisaccharide acceptor with a non-oxidized precursor of the glucuronic acid followed by post-glycosidation oxidation at the tetrasaccharide level and a subsequent [1+4] coupling reaction, was elaborated. In vitro evaluation of the anticoagulant activity of these new sulfonic-acid derivatives revealed that the disulfonate analogue inhibited the blood-coagulation-proteinase factor Xa with outstanding efficacy; however, the introduction of the third sulfonic-acid moiety resulted in a notable decrease in the anti-Xa activity. The difference in the biological activity of the disulfonic- and trisulfonicacid counterparts could be explained by the different conformation of their l-iduronic-acid residues.
N-acetilneuraminsav szulfonsav analogonjainak szintézise és influenzaellenes vizsgálata
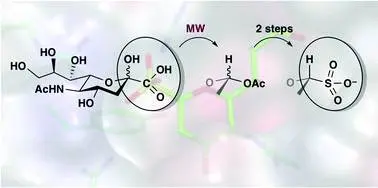
Ádám Hadházi, Mauro Pascolutti, Benjamin Bailly, Jeffrey C. Dyason, Anikó Borbás, Robin J. Thomson and Mark von Itzstein: A sialosyl sulfonate as a potent inhibitor of influenza virus replication
Org. Biomol. Chem., 2017, 15, 5249
Nukleozidkémiai kutatások
Triciklánók: új, triciklusos nukleozidszármazékok
Máté Kicsák, Attila Mándi, Szabolcs Varga, Mihály Herczeg, Gyula Batta, Attila Bényei, Anikó Borbás, Pál Herczegh: Tricyclanos: conformationally constrained nucleoside analogues with a new heterotricycle obtained from a d-ribofuranose unit.
Org Biomol Chem. 2018;16(3):393-401
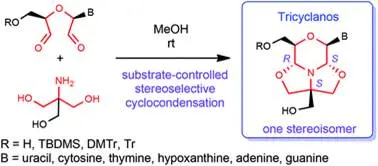
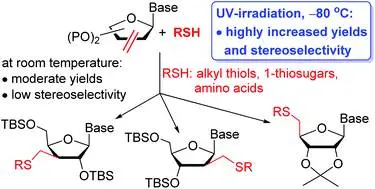
Ciszteinil nukleozid-monomerek előállítása és belőlük peptidláncú, töltésnélküli nukleinsavmimetikumok szintézise
Miklós Bege, Ilona Bereczki, Mihály Herczeg, Máté Kicsák, Dániel Eszenyi, Pál Herczegh and Anikó Borbás: A low-temperature, photoinduced thiol–ene click reaction: a mild and efficient method for the synthesis of sugar-modified nucleosides
Org. Biomol. Chem., 2017,15, 9226-9233
Egyéb
Aszpirin hibridek előállítása
Védőcsoport stratégiák kidolgozása
Máté Kicsák, Miklós Bege, Ilona Bereczki, Magdolna Csávás, Mihály Herczeg, Zoltán Kupihár, Lajos Kovács, Anikó Borbás, Pál Herczegh: A three-component reagent system for rapid and mild removal of O-, N- and S-trityl protecting groups.
Org. Biomol. Chem., 14 (12) (2016) 3190-3192.
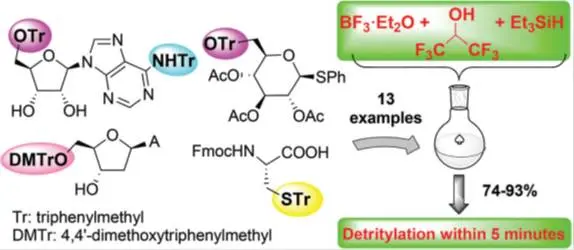
A new reagent system consisting of a Lewis acid such as BF3·Et2O or Cu(OTf)2, the mild protic acid hexafluoroisopropanol and the reducing quenching agent triethylsilane was elaborated for O-, N- and S-detritylation of nucleoside, carbohydrate and amino acid derivatives. The method is compatible with acetyl, silyl, acetal and Fmoc groups.
Aktuális pályázatok:
OTKA K109208 2013-2017.
Peptid-oligonukleozidok, oligomannozid mimetikumok és királis koronaéterek szintézise tio-click módszerrel
Vezető kutató: Dr. Borbás Anikó
NKFIH K16 K119509 2016-2020
Félszintetikus glikopeptidek és multivalens szénhidrátok szintézise és antibakteriális vizsgálata
Vezető kutató: Dr. Csávás Magdolna
NKFIH Indiai-magyar TÉT pályázat 2016-2020
Stabil glikomimetikumok és királis oxatia-koronaéter
Vezető kutató: Dr. Borbás Anikó
DECHEM GINOP-2.3.2-15-2016-00008 2016-2020
Kémia az életminőség javításáért: Stratégiai K+F Műhely a Debreceni Egyetemen
Résztvevő kutatók: Prof. Borbás Anikó, Prof. Herczegh Pál, Dr. Csávás Magdolna, Dr. Herczeg Mihály, Dr. Mező Erika, Dr. Kicsák Máté, Dr. Bege Miklós, Dr. Szűcs Zsolt, Eszenyi Dániel
PHARMPROT GINOP-2.3.2-15-2016-00044 2016-2020
A gyógyszerkutatás újabb irányai: peptid-fehérje kölcsönhatások a magasabb rendű fehérjeszerveződések szabályozásában
Résztvevő kutatók: Prof. Herczegh Pál, Prof. Borbás Anikó
OTKA PD 115645 2015-2018
Potenciálisan véralvadásgátló 6-dezoxi-L-talopiranóz tartalmú idraparinux analógok szintézise
Vezető kutató: Dr. Herczeg Mihály
NEMZETKÖZI KAPCSOLATOK
- Florent Perret
University Claude Bernard
Institut de Chimie et de Biochimie Moleculaire et Supramoléculaire
- Lenka Malinovska, Gita Jancarikova
Masaryk University
Central European Institute of Technology (CEITEC)
- Gabriela Novotna
Academy of Sciences of the Czech Republic
Institute of Microbiology
- Lieve Naesens
Rega Institute for Medical Research
- Margaret Dah-Tsyr Chang
National Tsing Hua University
Institute of Molecular and Cellular Biology
- Cheng Hsun-Chiu
Chang Gung University
Molecular Infectious Diseases Research Center, Chang Gung Memorial Hospital
- Andrey E. Shchekotikhin
Gause Institute of New Antibiotics Russian Academy of Sciences
Head of Laboratory of Chemical Transformations of Antibiotics
- Michaela Wimmerová
Masaryk University
National Centre for Biomolecular Research, Faculty of Science
- Yuan-Chuan Lee
Johns Hopkins University
Department of Biology
- Mark von Itzstein
Griffith University
Institute for Glycomics